Main Page: Difference between revisions
No edit summary |
No edit summary |
||
| (25 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<languages/> | |||
<translate> | |||
== Goal == <!--T:33--> | |||
</translate> | |||
[[File:Animation cycle recepteur ideal.gif|frame|right|<translate><!--T:34--> receiver cycle<br />(heat pump)</translate>]] | |||
<translate><!--T:35--> | |||
To develop a reversible [https://en.wikipedia.org/wiki/Heat_engine heat engine], which circulates a gas in a closed circuit between two insulating cylinders of different volumes, through two heat exchangers, in order to approach the [https://en.wikipedia.org/wiki/Carnot_cycle Carnot cycle]. | |||
<!--T:36--> | |||
This machine can be used to design heat pumps or refrigerators without greenhouse gases such as R93, as there is no phase change. It could also be used as a motor, producing mechanical energy by transferring heat from a hot source to a cold source. | |||
<!--T:37--> | |||
On a similar principle, the [https://en.wikipedia.org/wiki/Stirling_engine Stirling engine] has been around for two centuries, in this engine the cylinders are also the heat exchangers, the innovative idea here is to transfer heat as the gas passes from one cylinder to another. | |||
<!--T:38--> | |||
In the theoretical machine that [https://en.wikipedia.org/wiki/Nicolas_L%C3%A9onard_Sadi_Carnot Sadi Carnot] used to describe his ideal cycle, the hot and cold sources must act alternately in the same place, which seems technically unfeasible. The use of two cylinders and two heat exchangers makes it possible to imitate this machine, but induces friction and dead volume.</translate><br clear=all> | |||
<translate> | |||
== Ideal cycle == <!--T:39--> | |||
=== Motor cycle === <!--T:40--></translate> | |||
{| class="wikitable" | {| class="wikitable" | ||
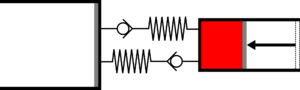
| | |<translate><!--T:41--> '''1 Adiabatic compression:''' The gas is compressed in the small cylinder, increasing its temperature.</translate> | ||
|[[File:moteur_compression_adiabatique.png|frameless|<translate><!--T:42--> the large cylinder remains empty</translate>]] | |||
|[[File: | |||
|- | |- | ||
|'''2 | |<translate><!--T:43--> '''2 Isothermal expansion:''' The gas expands from the small cylinder to the large cylinder, receiving heat in the first heat exchanger.</translate> | ||
|[[File: | |[[File:moteur_detente_isotherme.png|frameless|<translate><!--T:44--> the small cylinder empties completely while the large cylinder partially fills</translate>]] | ||
|- | |- | ||
|'''3 | |<translate><!--T:45--> '''3 Adiabatic expansion:''' The gas continues to expand in the large cylinder, reducing its temperature.</translate> | ||
|[[File: | |[[File:moteur_detente_adiabatique.png|frameless|<translate><!--T:46--> the small cylinder remains empty</translate>]] | ||
|- | |- | ||
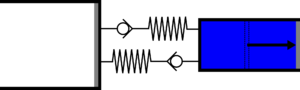
|'''4 | |<translate><!--T:47--> '''4 Isothermal compression:''' Gas is compressed from the large cylinder to the small one, and heat is extracted in the second heat exchanger.</translate> | ||
|[[File: | |[[File:moteur_compression_isotherme.png|frameless|<translate><!--T:48--> the large cylinder empties completely while the small one fills completely</translate>]] | ||
|} | |} | ||
---- | |||
<translate> | |||
=== Receiver cycle === <!--T:49--> | |||
</translate> | |||
{| class="wikitable" | {| class="wikitable" | ||
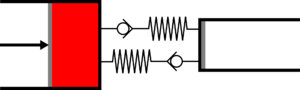
| | |<translate><!--T:50--> '''1 Adiabatic expansion:''' The gas is expanded in the small cylinder, reducing its temperature.</translate> | ||
|[[File:Recept_detente_adiabatique.png|frameless|<translate><!--T:51--> the large cylinder remains empty</translate>]] | |||
|[[File: | |||
|- | |- | ||
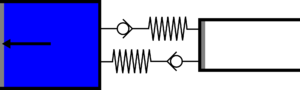
|'''2 | |<translate><!--T:52--> '''2 Isothermal expansion:''' The gas expands from the small cylinder to the large cylinder, capturing heat in the first heat exchanger.</translate> | ||
|[[File: | |[[File:Recept_detente_isotherme.png|frameless|<translate><!--T:53--> the small cylinder empties completely, while the large cylinder fills completely</translate>]] | ||
|- | |- | ||
|'''3 | |<translate><!--T:54--> '''3 Adiabatic compression:''' The gas is compressed in the large cylinder, increasing its temperature.</translate> | ||
|[[File: | |[[File:Recept_compression_adiabatique.png|frameless|<translate><!--T:55--> the small cylinder remains empty</translate>]] | ||
|- | |- | ||
|'''4 | |<translate><!--T:56--> '''4 Isothermal compression:''' The gas is compressed from the large cylinder to the small one, releasing heat in the second heat exchanger.</translate> | ||
|[[File: | |[[File:Recept_compression_isotherme.png|frameless|<translate><!--T:57--> the large cylinder empties completely while the small one partially fills</translate>]] | ||
|} | |} | ||
== | <translate> | ||
== Thermodynamics == <!--T:58--> | |||
== | |||
=== Operating temperatures === <!--T:59--> | |||
<!--T:60--> | |||
The extreme temperatures reached by the gas depend on the nature of the gas, its initial pressure, and the stroke of the pistons during adiabatic phases. A machine capable of modifying the latter parameter would allow working with variable temperatures. | |||
=== Heat transfer === <!--T:61--> | |||
<!--T:62--> | |||
The realization of isothermal phases in the ideal cycle requires the matching of : | |||
* the nature of the gas and its initial pressure, | |||
* the characteristics of the heat exchangers, the difference between the temperatures of the hot and cold sources and the extreme temperatures of the gas, | |||
* the volume ratio of the two cylinders. | |||
== Mechanics == <!--T:63--> | |||
<!--T:64--> | |||
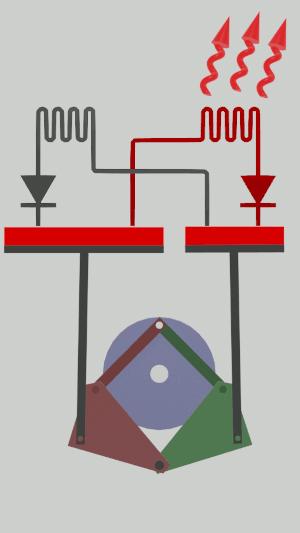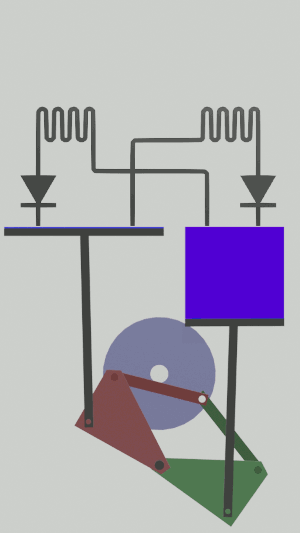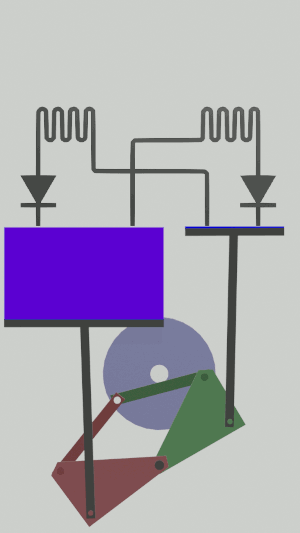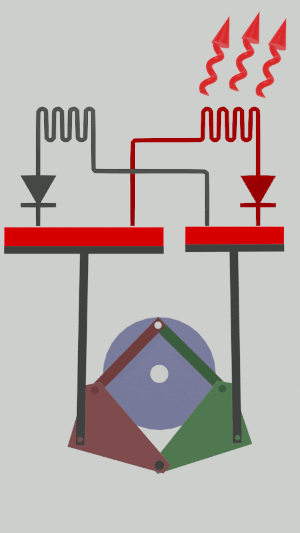
The technical possibilities for animating the pistons are numerous, particularly for the receiver cycle, solenoids, camshafts, cylinders … but a simple [[Special:MyLanguage/4-bar mechanism|4-bar mechanism]] seems promising for the realization of an efficient low tech heat pump:</translate>[[File:Animation recepteur 4 barres.gif|center|<translate><!--T:65--> Heat pump animated by two symmetrical 4-bar mechanisms</translate>]] | |||
<translate> | |||
== Is it free? == <!--T:66--> | |||
<!--T:67--> | |||
Totally! The description of this invention is published here under [https://creativecommons.org/publicdomain/zero/1.0/deed.en Creative Commons Zero] license, however I cannot guarantee that all or part of this machine is not currently protected by a patent. | |||
<!--T:68--> | |||
I sincerely hope that this idea will help reduce greenhouse gases emissions, and I'm convinced that making it free is the best thing I can do to facilitate its development and rapid spread. | |||
== You can help! == <!--T:69--> | |||
<!--T:70--> | |||
By contributing with your ideas, knowledge, translations, corrections to the wiki; and also links to your work in case you don't want to contribute under the CC0 license. | |||
Just register and edit. | |||
<!--T:71--> | |||
You can also make a donation. | |||
</translate> | |||
Latest revision as of 04:45, 23 November 2023
Goal

(heat pump)
To develop a reversible heat engine, which circulates a gas in a closed circuit between two insulating cylinders of different volumes, through two heat exchangers, in order to approach the Carnot cycle.
This machine can be used to design heat pumps or refrigerators without greenhouse gases such as R93, as there is no phase change. It could also be used as a motor, producing mechanical energy by transferring heat from a hot source to a cold source.
On a similar principle, the Stirling engine has been around for two centuries, in this engine the cylinders are also the heat exchangers, the innovative idea here is to transfer heat as the gas passes from one cylinder to another.
In the theoretical machine that Sadi Carnot used to describe his ideal cycle, the hot and cold sources must act alternately in the same place, which seems technically unfeasible. The use of two cylinders and two heat exchangers makes it possible to imitate this machine, but induces friction and dead volume.
Ideal cycle
Motor cycle
Receiver cycle
Thermodynamics
Operating temperatures
The extreme temperatures reached by the gas depend on the nature of the gas, its initial pressure, and the stroke of the pistons during adiabatic phases. A machine capable of modifying the latter parameter would allow working with variable temperatures.
Heat transfer
The realization of isothermal phases in the ideal cycle requires the matching of :
- the nature of the gas and its initial pressure,
- the characteristics of the heat exchangers, the difference between the temperatures of the hot and cold sources and the extreme temperatures of the gas,
- the volume ratio of the two cylinders.
Mechanics
The technical possibilities for animating the pistons are numerous, particularly for the receiver cycle, solenoids, camshafts, cylinders … but a simple 4-bar mechanism seems promising for the realization of an efficient low tech heat pump:
Is it free?
Totally! The description of this invention is published here under Creative Commons Zero license, however I cannot guarantee that all or part of this machine is not currently protected by a patent.
I sincerely hope that this idea will help reduce greenhouse gases emissions, and I'm convinced that making it free is the best thing I can do to facilitate its development and rapid spread.
You can help!
By contributing with your ideas, knowledge, translations, corrections to the wiki; and also links to your work in case you don't want to contribute under the CC0 license.
Just register and edit.
You can also make a donation.